Which board certified dental implant specialist
Why did the FDA ban silicone implants?
In the early 1990s, the FDA banned silicone breast implants in response to public concerns about health risks including cancer, connective tissue diseases, and autoimmune disorders. Read also : How long does dental implant surgery take. Later research found no link between breast implants and these diseases.
Can silicone implants cause cancer? Breast implants do not cause breast cancer. They don’t increase your chances of breast cancer, either. But research shows that women with breast implants have a higher chance of anaplastic large cell lymphoma (ALCL).
Are silicone breast implants Safe 2021?
Saline and silicone breast implants are considered safe for breast augmentation and breast reconstruction. To see also : Are there different types of dental implants. Research on the safety and effectiveness of both types of implants is ongoing.
Are silicone breast implants safer now?
According to current research, there are no significant differences in the safety of silicone gel and saline implants. But almost every type of implant has its advantages and disadvantages. Ruptures. Ruptures are a risk with either type of breast implant.
What is the new warning about breast implants?
FDA adds new restrictions, warning labels for breast implants following reports of cancer. The FDA added new restrictions on all breast implants on the market after years of tracking cases of rare cancers and reports of a constellation of symptoms that can include fatigue, memory loss, confusion, rashes and joint pain.
Why Are silicone breast implants toxic?
Silicone contains carcinogens and toxins that can wreak havoc in the body. For example, it can cause connective tissue diseases, inflammation and symptoms of breast implant illness. On the same subject : Does sucking your teeth hurt a dental implant. Women have reported a variety of symptoms, including sinus problems, allergies and dizziness, caused by a leaking silicone implant.
What are the side effects of silicone breast implants?
These include:
- bruise
- bleeding
- blood clots.
- skin necrosis.
- slow wound healing.
- accumulation of scar tissue (capsular contracture)
- implant deflation and rupture.
- change in breast shape, volume, or sensation.
What toxins are in silicone implants?
The concern is that some chemicals containing platinum in certain charged forms (ie oxidation states) are known to be toxic (eg, cause allergic effects). Claims have been made that one of these chemicals, called hexachloroplatinate (or platinate), is present in silicone breast implants.
Are silicone implants approved by the FDA?
The FDA has also approved breast implants to correct or improve the result of previous surgery. There are two types of FDA-approved breast implants: saline (a saltwater solution) and silicone gel.
When did the FDA approve silicone breast implants?
In November 2006, the FDA approved two implants almost entirely filled with silicone gel: the Allergan Natrelle and the Mentor MemoryGel. As conditions of approval, the FDA required all manufacturers to conduct post-approval studies to characterize the devices’ long-term performance and safety.
Are silicone implants legal?
Hi, it is legal and possible to have implants filled with silicone gel at your age. This is called ‘off label’ use, and it is perfectly legal. You and your surgeon are doing nothing wrong, and the warranty on the implants will be honored.
Do dental products need FDA approval?
If your dental laboratory manufactures Class I or Class II devices, such as sleep apnea, anti-snoring devices, or TMJ splints, then you must register with FDA. If you or your business owns a 510(k), then you are considered a manufacturer and must register with the FDA.
Do toothbrushes need FDA approval? A manual toothbrush is 510(k) exempt, meaning it does not require premarket notification or approval.
Who regulates dental materials?
The ADA sets standards for almost all dental instruments, ensuring their safety, reliability and effectiveness for dentists and the public. Over 600 volunteers from a wide range of interests contribute their expertise.
Who regulates dental materials in Canada?
Canadian Health Care System This also means that the regulation of health professionals such as dentists is a provincial/territorial responsibility.
Does the FDA regulate dental materials?
The FDA does not regulate dentists or dictate what products a dentist can use; it regulates industry and products that fall under its jurisdiction.
Are dental materials FDA approved?
Many factors go into the ways in which the FDA approves medical and dental materials and devices. They are entrusted with protecting the health and welfare of our citizens in many ways including the safety of medical and dental materials and devices used in this country.
Does the FDA regulate dental materials?
The FDA does not regulate dentists or dictate what products a dentist can use; it regulates industry and products that fall under its jurisdiction.
Are Dental Implants FDA approved?
All the implants that are going to be used in this practice are FDA approved, and have gone through a great deal of scientific control study for use in the human body.
Does the FDA regulate dental devices?
For dentists and dental laboratories, the raw materials and equipment/technology used to provide dental services are regulated in some way by the US Food and Drug Administration (FDA).
Does the FDA regulate dental materials?
The FDA does not regulate dentists or dictate what products a dentist can use; it regulates industry and products that fall under its jurisdiction.
What devices are regulated by the FDA?
| Controlled Product | Find information about: |
|---|---|
| Medical Devices | Medical devices such as bandages, contact lenses, first aid kits, pacemakers and surgical instruments |
| Electronic Products Emitting Radiation | Products that emit radiation such as x-ray machines, microwave ovens, CD-ROMs, LEDs, and laser pointers |
How long does your mouth hurt after a dental implant?
You May Experience Pain & Other Symptoms For Up To 7 Days After about 3-7 days, you will likely still feel some pain and tenderness around the implant site. However, it should start to become less painful. You can usually return to work or school within 1-3 days after your operation.
Why does my dental implant still hurt? The most common reason for prolonged pain after dental implants is infection at the implant site. If the implant site is not properly cleaned and disinfected, it can become inflamed, causing pain and discomfort, and prevent the implant from healing properly.
How long will dental implant pain last?
How long will it take for pain from an implant to subside? In most cases, the discomfort will peak within about 3-5 days after your treatment, and then begin to subside relatively quickly. By the end of your first week after surgery, you should be feeling little or no discomfort and pain.
Why does my implant hurt after 2 weeks?
Continued pain after two weeks with Your implant site should feel almost completely normal after two weeks. If you continue to feel pain after this time, seek a follow-up appointment immediately. The site looks inflamed or infected with If the gums near the implant site look grey, brown or black, this may be a sign of a serious infection.
What is normal pain after dental implants?
You should not feel any severe pain or discomfort, and there will be no bleeding and little to no swelling or bruising around the area. By this time, you can resume vigorous physical activities such as running and resume your normal diet. Severe pain and discomfort is rare after two weeks.
How can I ease the pain of dental implants?
What to expect after dental implant surgery
- Taking Medicines: Painkillers, known as analgesics, are the most effective and immediate way to reduce post-surgical discomfort and pain. …
- Use an Ice Pack or Cold Compress: The use of a cold compress is generally recommended as a way to reduce early swelling and bruising.
How long does it take for gums to heal after dental implants?
The gum will start to heal after about three days. There will be a full recovery after one to two weeks. Another pre-implant restoration procedure is bone grafting. Some patients need this if there is significant loss of jawbone.
How long does it take for a dental implant to feel normal?
Depending on how quickly you heal, your mouth will start to feel normal again about 1-2 weeks after your implant placement surgery. At this stage, you should feel no more pain, and you can eat your normal diet and resume vigorous activities such as exercise.
Do gums heal over implants?
As you recover from dental implants, your gums will gradually grow around the dental implants to provide support as they do for your natural teeth. However, your dentist will also monitor your gum growth during your healing and recovery process to ensure that the gums do not completely overgrow the implant.
How painful is it to have implants?
A simple dental implant, for a patient with good bones and not requiring a lot of soft tissue surgery, has a pain level of two to three in the first 24 to 48 hours, which means over the counter medication like Tylenol or Advil will do takes care of any discomfort they feel.
How does it feel to have an implant? You will not feel any discomfort, as the area is completely anaesthetised. After making the area more accessible, a hole can be drilled for the implant. Although drills may also sound painful, your jawbone does not have nerves to feel any pain. The biggest discomfort you might feel is pressure.
How long does pain last after tooth implant?
You May Experience Pain and Other Symptoms for Up to 7 Days After about 3-7 days, you will likely still feel some pain and tenderness around the implant site. However, it should start to become less painful. You can usually return to work or school within 1-3 days after your operation.
Why is my new dental implant throbbing?
During the healing process, you will experience the pain for the first few days. The pain is not so intense, and can be controlled using some painkillers prescribed by the doctor. In the event of a dental implant failure, you will experience excruciating pain and discomfort that comes in the form of throbbing waves.
How can I stop my implant from hurting?
Tips to reduce dental implant pain
- 1) Take complete rest and refrain from strenuous activities. …
- 2) Take medicines and painkillers. …
- 3) Cold Compression. …
- 4) Soak your mouth with warm water and baking soda solution. …
- 5) Stop the habit of smoking. …
- 6) Switch to soft foods.
What hurts more tooth extraction or implant?
It is suggested that pain intensity is higher with tooth extraction compared to the implant placement procedure.
Are dental implants extremely painful?
It is common for patients to experience some pain after the dental implant procedure. Initially, the discomfort may last one or two days. However, some patients may continue to experience pain at the implant site for up to 10 days.
Can a tooth be pulled and implant the same day?
Same Day Dental Implants With same day implants, your surgeon will remove the problem tooth and place an implant in the extraction site on the same day. This treatment has significantly reduced the waiting period, allowing patients to have their dental problems fixed as quickly as possible.
Does Tufts Dental School do implants?
Tufts University School of Dental Medicine is offering a new two-year Certificate program in Implant Dentistry.
.
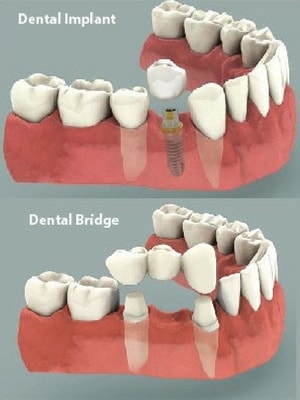
Are dental implants considered medical devices?
Dental implants are medical devices that are surgically implanted into the jaw to restore a person’s ability to chew or their sight. They provide support for artificial (false) teeth, such as crowns, bridges, or dentures.
Are dentures considered medical devices? Taxable medical devices include dentures, bridges, implant abutments, crowns, inlays, onlays, veneers, orthodontic appliances, sleep apnea, etc. The tax also provides no exemption for dentists who have in-house laboratories or who manufacture devices using CAD/CAM in the office.
What are implantable medical devices examples?
The list of most commonly used implantable medical devices includes artificial joints, breast implants, contraceptive Intrauterine Devices (IUDs), and bone, muscle and joint fusion hardware.
What are implantable medical devices?
A medical device is defined as one that can be implanted if it is introduced partially or completely, surgically or medically, into the human body and is intended to remain there after the treatment [1-2].
What is an example of a medical device?
Examples are ultrasound and MRI machines, PET and CT scanners, and x-ray machines. Treatment equipment includes infusion pumps, medical lasers and LASIK surgical machines. Life support equipment is used to maintain a patient’s physical function.
What class of device are dental implants?
Dental implants and dental implant abutments are currently class IIb medical devices under the Medical Devices Directive (MDD, 93/42/EEC), and this classification will not change under the MDR.
What are dental implants classified as?
Dental Implants Are Part Of Prosthodontics This includes things like dental crowns and bridges, dentures, and even dental implants. As dental implants are intended to replace one or more missing teeth, they are firmly established within the specialty of prosthodontics.
Are orthopedic implants Class 3?
Class III is the most scientifically rigorous classification of medical devices and encompasses most of the orthopedic implants on the market today.
Are implants Class III medical devices?
Class III a These devices are usually life-sustaining or life-sustaining, implanted, or present an unreasonable potential risk of illness or injury. Examples of Class III devices include implantable pacemakers and breast implants. 10% of medical devices fall under this category.
What are Class 3 medical devices examples?
Examples of Class III Medical Devices:
- Breast implants.
- Accelerators.
- Defibrillators.
- High frequency ventilators.
- Cochlear implants.
- Monitors fetal blood sampling.
- Implanted prosthetics.
Are implants considered a medical device?
An implant is a medical device manufactured to replace a missing biological structure, to support a damaged biological structure, or to improve an existing biological structure. Medical implants are man-made devices, unlike a transplant, which is transplanted biomedical tissue.



Comments are closed.